Sodium Hypochlorite vs Hypochlorous Acid
Introduction
When it comes to effective disinfecting, chlorine-based compounds have been the go-to solution for decades. But not all chlorine forms are created equal. At Shandong Shine Health Co., Ltd., we’re often asked about the differences between hypochlorous acid (HOCl) and sodium hypochlorite (bleach) and which one works better. The answer lies in chemistry—and a touch of biology.
1. What’s Free Available Chlorine (FAC)?
Free Available Chlorine (FAC) is the disinfecting form of chlorine. FAC refers to both hypochlorous acid (HOCl) and hypochlorite (OCl-) ions, but these two differ greatly in their power to kill germs. HOCl, for example, is estimated to be 80 to 120 times more effective than sodium hypochlorite, and here’s why.
2. Molecular Charge: The Secret to HOCl’s Power
· Neutral vs. Negative Charges: HOCl molecules have a neutral charge, while sodium hypochlorite has a negative charge. Since many bacteria and viruses have negatively charged cell walls, sodium hypochlorite is repelled, like magnets with similar poles.
· Hypochlorous Acid as a “Trojan Horse”: Because HOCl isn’t repelled by negatively charged microbes, it penetrates cell walls and dismantles them from within.
3. Chlorine and pH Values: A Balancing Act
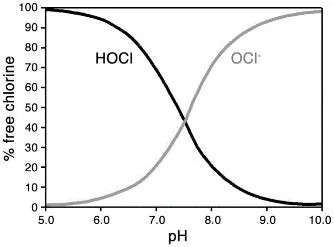
· pH’s Influence on Chlorine Forms: In any solution, chlorine forms change based on pH. HOCl is the dominant form at lower pH values, which makes it more effective at disinfection.
· High pH in Bleach: To increase shelf life, bleach is stabilized with sodium hydroxide, giving it a pH around 13. While this high pH preserves bleach, it also makes it harsh on surfaces and dangerous for skin contact.
4. Why Hypochlorous Acid Mimics Nature’s Immune System
Our bodies naturally produce HOCl in white blood cells to combat infections. HOCl is a safe, effective compound that won’t harm healthy cells, unlike harsh bleach solutions. Using HOCl is like enhancing the immune system's natural response to fight off germs safely.
5. Sodium Hypochlorite’s Heavy-Duty Power
Sodium hypochlorite is powerful but can be dangerous. Bleach’s pH is dangerously high, making it corrosive and irritating. When disinfecting requires aggressive power, bleach can work—but with caution.
6. How pH Affects HOCl’s Efficiency
Since HOCl is most effective at a pH of 5-6, it aligns closely with a natural, neutral pH, fooling microbes into “drinking” it. This allows it to penetrate cells easily and kill from within, making it incredibly effective against bacteria and viruses.
7. Comparing Effectiveness Against Germs
Both HOCl and bleach can kill a wide range of bacteria and viruses, but HOCl’s neutral pH and high penetration rate make it especially suited for sensitive settings like wound care and home cleaning.
8. HOCl and Sodium Hypochlorite in Wound Care
HOCl’s neutral nature makes it ideal for wound care, offering effective germ control without irritating sensitive tissues. Bleach, on the other hand, is too harsh for such uses, potentially causing damage and stinging.
9. Environmental and Health Safety
· HOCl’s Biodegradability: Hypochlorous acid breaks down into harmless byproducts, making it environmentally friendly.
· Bleach’s Environmental Impact: Sodium hypochlorite, however, can release harmful byproducts that negatively impact ecosystems, water systems, and air quality.
10. Practical Use for Home Cleaning Products
For everyday disinfecting, HOCl wins as a gentler option. While bleach is economical and effective, especially in industrial settings, HOCl’s neutral pH and safety make it a favorite for home use.
11. Toxicity Considerations: Human Health First
HOCl is a top choice for spaces where respiratory health is essential, as it releases fewer fumes. Bleach, however, should be handled with caution to avoid irritation to the respiratory system and skin.
12. Immune System's Own Hypochlorous Acid
Our white blood cells produce HOCl to fight infections naturally. This natural disinfectant reflects why HOCl-based products are increasingly popular—they work in harmony with the body’s own defense mechanisms.
13. FAQs About HOCl and Bleach
· Can HOCl Be Used in Food Prep? Yes, it’s non-toxic and safe for food surfaces.
· Is Bleach Effective in Low Concentrations? Lower concentrations can be less effective but are safer for home use.
Conclusion
Hypochlorous acid, with its neutral pH and safety for the environment, stands as the gentler disinfecting choice, mimicking the body’s immune response. While bleach has a place in heavy-duty cleaning, HOCl offers powerful disinfection without the risks.
References
1. National Center for Biotechnology Information
2. CDC Disinfection Guidelines
