
- HOME
- Products
- Sodium Hypochlorite Generator
- Sodium hypochlorite generator chemical reaction formula
The chemical reaction of the sodium hypochlorite generator is stable, ensuring its disinfection and sterilization effect.
Effective chlorine production: 50-400000g/H
Effective chlorine concentration: 3-10g/L
Installed power: 0.3-90KW
Host size: customized
Delivery cycle: 25 days
WhatsApp:+86 19953182842
Email: henry@hoclshine.com
Product Details
The chemical reaction of the sodium hypochlorite generator is stable, and the sodium chloride solution undergoes a series of electrochemical reactions
in the electrolytic cell under the action of a certain electrode, generating the sodium hypochlorite generator solution.Product parameter
Effective chlorine production: 50-400000g/H
Effective chlorine concentration: 3-10g/L
Installed power: 0.3-90KW
Host size: customized
Delvery cycle: 25 days
WhatsApp:+86 19953182842
Email: henry@hoclshine.com
Chemical reaction formula
Anodic reaction: 2Nacl → 2Na++Cl+2e-
Cathodic reaction: 2H2O+2e - → H2 ↑+2OH-
Extreme reaction:2NaOH+Cl2→NaCLO+NaCL+H2O
Total reaction:Nacl+H2O→NaCLO+H2 ↑
Because the molecular weight of Naclo is 1.05 times that of CL2, and during the oxidation reaction, the number of charge transfers between each
Naclo molecule and CL2 molecule is the same. Therefore, in the process of producing sodium hypochlorite generator through electrochemical reaction,
every 1g of Naclo generated is equivalent to 0.952g of Cl2.
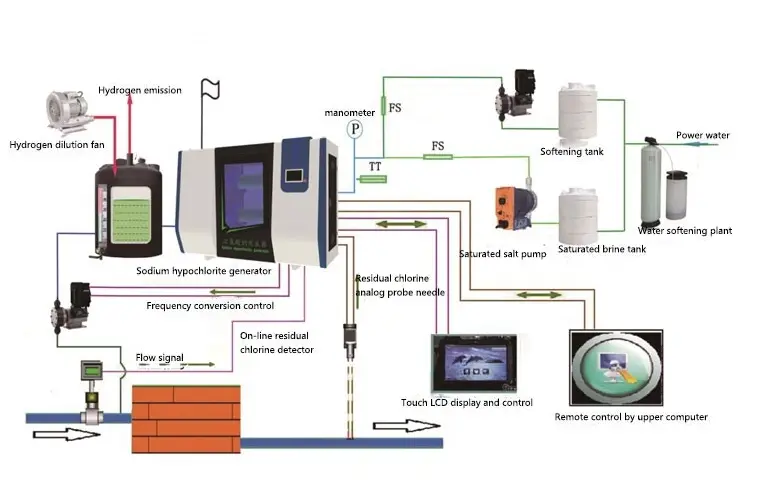
Technological process
Application scenarios

Submitted successfully
We will contact you as soon as possible