
- HOME
- Products
- Sodium Hypochlorite Generator
- Sodium Hypochlorite Generator in Food Industry
Sodium hypochlorite (NaOCl) is a yellow green liquid formed by the combination of chlorine gas and sodium hydroxide. Commercial sodium hypochlorite is produced by adding chlorine gas to caustic soda, producing sodium hypochlorite, water, and salt. Alternatively, when a small amount is required, on-site production of sodium hypochlorite can be achieved by dissolving the salt in softened water. This will produce concentrated saline water, which can be electrolyzed to form a solution of sodium hypochlorite.
Effective chlorine production: 50-15000g/H
Effective chlorine concentration: 3-10g/L
Installed power: 0.3-90KW
Host size: Customized delivery cycle: 25 days
WhatsApp: +86 19953182842
Email: henry@hoclshine.com
Product Details
Sodium hypochlorite has many applications in agriculture, food, and beverage industries. Sodium hypochlorite maintains water quality through oxidation or disinfection. It can eliminate harmful microorganisms and prevent algae or shellfish from growing in stored water. It is an important component of many disinfection processes, including cleaning fruits and vegetables, as well as preparing meat and fish for consumption. As it is the main component of household bleach, it is used for cleaning and disinfecting equipment in factories and farms. In some countries, it is widely used for disinfection of drinking water and wastewater. It is an effective treatment method for private water supply from boreholes (also known as wells), where water is stored before industrial use.

Product parameter
Effective chlorine production: 50-15000g/H
Effective chlorine concentration: 3-10g/L
Installed power: 0.3-90KW
Host size: Customized delivery cycle: 25 days
WhatsApp: +86 19953182842
Email: henry@hoclshine.com
Product advantage
Sodium hypochlorite has a similar effect to chlorine and is a disinfectant that is highly effective against viruses, bacteria, and fungi because it can remove the cell walls of microorganisms, rendering nucleic acids useless and preventing the reproduction of these organisms.When added to water, it forms hypochlorous acid (HOCl) and chlorite ions (OCl -), also known as free chlorine.
The ratio between HOCl and OCl - depends on the pH value and temperature of the water. At 25 ° C and pH 7.5, chlorine is evenly distributed between
HOCl and OCl -. As part of this process, it may be necessary to acidify the water to obtain the desired ratio - ideally adding more hypochlorous acid to
the mixture as it is the best disinfectant. Due to the negative charge of hypochlorite ions, they will bounce off the walls of microbial cells that are also
negatively charged. The chlorite ion is also larger than the HOCl molecule, so the diffusion rate is slower. Hypochloric acid is usually the best choice
because it is a stronger oxidant than hypochlorite ions.
Hypochloric acid rapidly degrades when in contact with organic matter, therefore it does not have a lasting impact on the environment.
Like other forms of chlorine, it is necessary to regularly test the pH and chlorine content of treated water to ensure compliance with local quality standards.
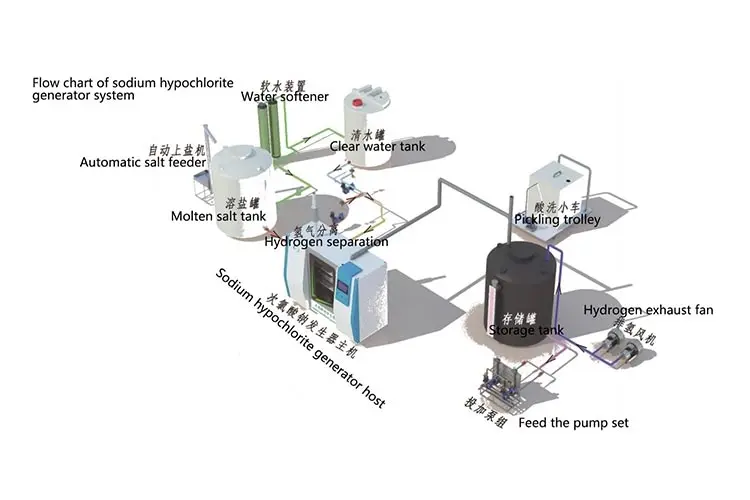
Process Flow Diagram
Qualification certificate

Application

Customer negotiation



Submitted successfully
We will contact you as soon as possible