
- HOME
- Products
- Sodium Hypochlorite Generator
- Working principle of sodium hypochlorite generator with effective chlorine concentration of 0.3% -1%
The main reaction process of sodium hypochlorite generator electrolysis can be represented by the following equation: NaCl+H2O=NaClO+H2 ↑
Effective chlorine production: 50-400000g/H
Effective chlorine concentration: 3-10g/L
Installed power: 0.3-90KW
Host size: customized
Delivery cycle: 25 days
WhatsApp:+86 19953182842
Email: henry@hoclshine.com
Product Details
The electrolysis process of an electrolytic salt water type sodium hypochlorite generator is an electrochemical reaction process. Its only raw material is salt water, with no additional components, and the quality of the sodium hypochlorite solution produced is pure.
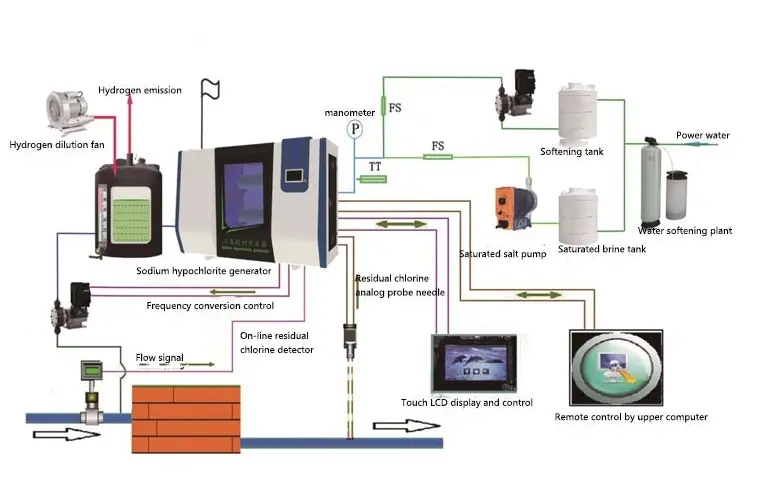
Product parameter
Effective chlorine production: 50-400000g/H
Effective chlorine concentration: 3-10g/L
Installed power: 0.3-90KW
Host size: customized
Delivery cycle: 25 days
WhatsApp:+86 19953182842
Email: henry@hoclshine.com
Product advantage
1. Its working principle is that under a certain cell voltage, a sodium chloride solution undergoes a series of electrochemical reactions in the electrolytic cell, ultimately generating a sodium hypochlorite solution.
2. Because the molecular weight of NaCLO is 1.05 times that of Cl2, and during the oxidation reaction, the number of charge transfers between each NaCLO molecule and Cl2 molecule is the same. Therefore, in the process of producing sodium hypochlorite through electrochemical reaction, every 1 gram of NaCLO produced is equivalent to 0.952 grams of Cl2.
3. The process of generating sodium hypochlorite simultaneously produces a considerable amount of hydrogen gas. Because in the process design, a centrifugal fan is used to mix fresh air with the generated hydrogen gas, the calculation of hydrogen gas quantity is based on the maximum input current, where the cathode produces 100% hydrogen gas. According to this calculation, approximately 0.35 liters of H2 are generated for every 1 gram of NaCLO produced.
Qualification certificate


Submitted successfully
We will contact you as soon as possible