What is the common name for HOCl?
Introduction:
Have you ever wondered what HOCl is commonly called? You're not alone! This incredible compound plays a huge role in health, disinfection, and water treatment.
We interact with it more often than we might realize, especially when it comes to sanitation and public health. In this guide, we’ll explore the common name for HOCl, its chemistry, and much more.
This article is packed with details, breaking down everything from the pKa of HOCl to its molecular model, oxidation number, and chemical properties.
And yes, we’re keeping it simple and clear! Let’s dive into the world of HOCl and uncover why it’s such a big deal.
Table of Contents:
What is HOCl?
The Common Name for HOCl
Chemical Properties of HOCl
The pKa of HOCl: What Does It Mean?
Understanding the Molecular Model of HOCl
HOCl as an Acid: HOCl Acid Name
HOCl's Role as an Oxidizing Agent
HOCl in Water Treatment
HOCl's Oxidation Number: Breaking It Down
How HOCl Reacts in Different pH Levels
Applications of HOCl in Health and Safety
Common Uses for HOCl in Everyday Life
How to Safely Generate HOCl On-Site
HOCl vs. Other Disinfectants: What’s the Difference?
Benefits of HOCl in the Medical Industry
The Future of HOCl in Disinfection
FAQs About HOCl
Summary and Final Thoughts
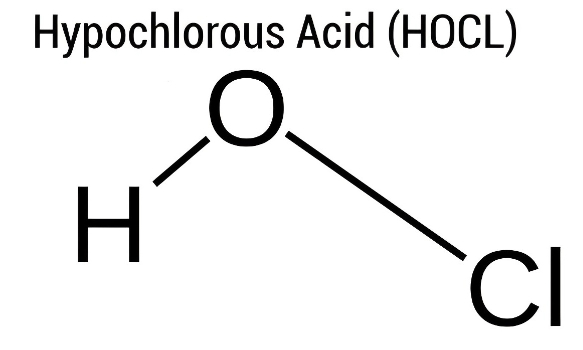
1. What is HOCl?
Let’s start with the basics. HOCl stands for hypochlorous acid, a compound we find in many disinfection products. It’s a weak acid formed when chlorine dissolves in water. While it may sound technical, HOCl is a natural and safe disinfectant that our own immune system produces to fight infections.
2. The Common Name for HOCl
The most common name for HOCl is hypochlorous acid. However, people may refer to it simply as chlorine-based disinfectant when they talk about its role in cleaning and sanitation. It’s often used interchangeably with other chlorine products, but hypochlorous acid has unique properties that set it apart from the rest.
3. Chemical Properties of HOCl
HOCl is known for its chemical simplicity: it consists of one hydrogen (H), one oxygen (O), and one chlorine (Cl) atom. This simplicity makes it a powerful yet gentle disinfectant. Its ability to neutralize harmful microorganisms while being safe on human skin is a huge advantage in industries like healthcare and food production.
4. The pKa of HOCl: What Does It Mean?
Understanding the pKa of HOCl helps us know how it behaves in different environments. pKa refers to the strength of an acid, and for HOCl, the value is around 7.5. This means that HOCl is most effective at a neutral pH level. If the pH shifts, it may become less effective, which is why controlling the pH in water treatment and disinfection is crucial.
5. Understanding the Molecular Model of HOCl
The molecular model of HOCl shows the arrangement of atoms: hydrogen bonded to oxygen, and oxygen bonded to chlorine. This structure is what gives hypochlorous acid its chemical reactivity. The simplicity of this model hides the strength behind HOCl’s ability to break down harmful microbes and protect against infection.
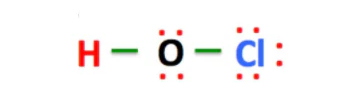
6. HOCl as an Acid: HOCl Acid Name
HOCl is a weak acid. Its acid name is hypochlorous acid. In chemical terms, this means that it can donate a hydrogen ion (H+) in reactions. In practical terms, this makes HOCl a fantastic cleaning agent that is strong enough to sanitize but gentle enough to use in sensitive environments like hospitals.
7. HOCl's Role as an Oxidizing Agent
One of HOCl’s most important properties is its ability to act as an oxidizing agent. This means it can accept electrons during chemical reactions, which helps it break down organic material and kill bacteria and viruses. It’s this oxidative property that makes HOCl so effective in sanitizing water and surfaces.
8. HOCl in Water Treatment
In water treatment, HOCl is a superstar. Its ability to kill pathogens while being safe for human consumption makes it ideal for disinfecting drinking water. It’s used in both small-scale applications, like household disinfectants, and large-scale operations, like municipal water treatment plants. The fact that HOCl doesn’t leave harmful residues is another big plus.

9. HOCl's Oxidation Number: Breaking It Down
The oxidation number of HOCl is +1 for chlorine. This reflects how chlorine in HOCl interacts during chemical reactions. It may sound technical, but this number is key in understanding how hypochlorous acid works as a disinfectant. It explains how HOCl can oxidize and destroy harmful bacteria, keeping environments clean and safe.
10. How HOCl Reacts in Different pH Levels
pH plays a big role in how effective HOCl is. At a neutral pH (around 7), HOCl is in its most active form. However, when the pH shifts to more acidic or alkaline conditions, HOCl starts to lose its disinfecting power. This is why pH control is essential in processes like water treatment, where HOCl must be at the right pH to be effective.
11. Applications of HOCl in Health and Safety
HOCl is used widely in healthcare settings because it’s non-toxic and safe for human tissues. From wound care to surface disinfection, hypochlorous acid provides powerful protection without the harsh chemicals found in other disinfectants. This makes it a top choice in hospitals, clinics, and even veterinary medicine.
12. Common Uses for HOCl in Everyday Life
You might not realize it, but HOCl is around us in many everyday products. From cleaning wipes to wound care solutions, hypochlorous acid helps keep our environments clean and safe. It’s found in products used for food safety, sanitation, and even skincare. Because it’s gentle on skin, it’s gaining popularity in personal hygiene products as well.
13. How to Safely Generate HOCl On-Site
Generating HOCl on-site is a cost-effective and sustainable option for businesses. Using an HOCl generator, companies can create fresh hypochlorous acid as needed, reducing the need for storage and transportation of chemical disinfectants. It’s a greener, safer, and more efficient way to maintain hygiene and sanitation.
14. HOCl vs. Other Disinfectants: What’s the Difference?
HOCl stands out compared to other disinfectants like bleach or alcohol. While bleach is harsh and can irritate skin and eyes, hypochlorous acid is gentle and safe for sensitive environments. It’s also more effective at killing certain pathogens than alcohol-based disinfectants. Plus, HOCl breaks down quickly, leaving no harmful residues.
15. Benefits of HOCl in the Medical Industry
In the medical industry, HOCl is valued for its ability to disinfect without causing harm to human tissues. It’s used in wound care products to clean and promote healing, and it’s also effective in sanitizing medical equipment. HOCl’s non-toxic nature makes it a safer alternative to traditional disinfectants that can cause irritation or allergic reactions.
16. The Future of HOCl in Disinfection
As the world becomes more focused on safe, sustainable disinfection methods, HOCl is gaining attention as a future-forward solution. Its versatility, safety, and effectiveness are making it a go-to choice for industries ranging from healthcare to hospitality. With ongoing research, we can expect to see even more innovative uses for hypochlorous acid in the coming years.
17. FAQs About HOCl
What is the common name for HOCl?
The common name is hypochlorous acid.
What is the pKa of HOCl?
The pKa of HOCl is approximately 7.5.
How does HOCl compare to bleach?
HOCl is gentler and safer than bleach, making it better for sensitive environments.
What is the molecular model of HOCl?
The molecular model of HOCl consists of hydrogen, oxygen, and chlorine atoms.
18. Summary and Final Thoughts
HOCl, commonly known as hypochlorous acid, is a powerful yet gentle disinfectant. From its role in water treatment to healthcare, this compound offers a versatile and effective solution for a variety of sanitation needs. Whether you’re generating it on-site or using it in everyday products, HOCl is proving to be an essential tool for clean, safe environments.
