Hypochlorous Acid: Strong or Weak?
Introduction
When we think of acids, we often envision something that can corrode metal or cause skin burns. But what about hypochlorous acid (HClO)? Is it strong or weak?
In this extensive article, we explore the properties of HClO, its benefits, applications, and how it plays a vital role in our daily lives.
Join us as we dive into the chemistry of HClO and uncover why it deserves our attention.
What is Hypochlorous Acid?
Hypochlorous acid is a simple compound formed when chlorine gas dissolves in water. It’s a key player in various applications, particularly in sanitation and disinfection.
· Chemical Composition: HClO consists of one hydrogen atom, one chlorine atom, and one oxygen atom.
· Formation: You create HClO when you mix chlorine gas with water. The reaction can be summarized as follows:
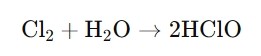
This shows how chlorine interacts with water to form hypochlorous acid.
Electrolysis: The Production of Hypochlorous Acid
One of the most efficient methods for producing hypochlorous acid (HClO) is through electrolysis. This process involves passing an electric current through a saltwater solution, resulting in the generation of HClO along with other byproducts. Let's delve deeper into how this process works and its significance.
What is Electrolysis?
Electrolysis is a chemical process that uses electrical energy to drive a non-spontaneous reaction. In the case of HClO production, it involves the electrolysis of a sodium chloride (NaCl) solution, commonly known as saline.
The Electrolysis Process
1. Preparation of the Solution:
· Mix water with a small amount of salt (sodium chloride). This creates a saline solution that will conduct electricity.
2. Electrolysis Setup:
· The saline solution is placed in an electrolytic cell, which contains two electrodes: an anode (positive electrode) and a cathode (negative electrode).
3. Applying Electric Current:
· When an electric current is applied, a series of electrochemical reactions occur at the electrodes.
4. Chemical Reactions:
· At the anode, chloride ions (Cl⁻) are oxidized to chlorine gas (Cl₂):
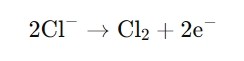
· At the cathode, water is reduced, producing hydrogen gas (H₂) and hydroxide ions (OH⁻):
![]()
5. Formation of Hypochlorous Acid:
· The chlorine gas produced at the anode reacts with water to form hypochlorous acid:
![]()
· The resulting solution contains HClO and other species, depending on the conditions of the electrolysis.
Benefits of Electrolysis for HClO Production
1. On-Site Generation:
· Electrolysis allows for the on-site production of HClO, ensuring freshness and reducing the need for storage.
2. Customizable Concentrations:
· Users can adjust the concentration of HClO by modifying the salt concentration and the duration of electrolysis.
3. Eco-Friendly:
· The process generates minimal waste, and the primary byproducts (water and salt) are environmentally friendly.
4. Cost-Effective:
· Producing HClO through electrolysis can be more economical than purchasing pre-made solutions, especially for large-scale applications.
Applications of Electrolytic HClO
The hypochlorous acid generated through electrolysis has various applications, making it a versatile solution in different industries.
1. Healthcare Settings
In hospitals and clinics, electrolytic HClO is used for disinfecting surfaces and medical equipment. Its effectiveness against a wide range of pathogens helps in infection control.
Food processing plants utilize electrolytic HClO for sanitizing equipment and surfaces, ensuring food safety by minimizing microbial contamination.
3. Water Treatment
Municipal water treatment facilities employ HClO generated from electrolysis to disinfect drinking water and wastewater, providing safe water for communities.
4. Household Cleaning
Many household cleaners and sanitizers now include electrolytic HClO, providing a safe and effective means to disinfect surfaces at home.
Understanding Acidity
To determine whether HClO is strong or weak, we need to understand the concept of acidity in chemistry.
The Definition of Acids
In chemistry, acids are defined by their ability to donate protons (H⁺ ions) to other substances.
· Brønsted-Lowry Theory: According to this theory, an acid is any substance that can donate a proton.
· Arrhenius Theory: This theory states that an acid is a substance that increases the concentration of hydrogen ions in aqueous solution.
Using these definitions, we can evaluate the behavior of hypochlorous acid.
Is HClO a Strong Acid?
Let’s clarify what makes an acid strong or weak.
· Strong Acids: Strong acids completely dissociate in water. This means they release all their hydrogen ions into the solution.
· HClO Behavior: Hypochlorous acid only partially dissociates in water, releasing some but not all hydrogen ions. As a result, we classify it as a weak acid.
This distinction is crucial, as the strength of an acid affects its reactivity and applications.
The pH of Hypochlorous Acid
The pH scale measures how acidic or alkaline a solution is, ranging from 0 (very acidic) to 14 (very alkaline).
· Typical pH of HClO: Hypochlorous acid usually has a pH around 5 to 6.5, indicating mild acidity.
Why Does pH Matter?
Understanding the pH of HClO helps us gauge how it interacts in various environments.
· Impact on Efficacy: The effectiveness of HClO as a disinfectant increases in certain pH ranges. For example, lower pH levels enhance its antimicrobial properties.
· Applications in Water Treatment: Maintaining the right pH in swimming pools ensures HClO remains effective in killing harmful microorganisms.
Benefits of Hypochlorous Acid
What makes HClO special? Here are some of its key benefits:
1. Effective Disinfectant: HClO is known for its ability to kill bacteria and viruses. This makes it a popular choice for sanitation in various settings.
2. Safe for Use: Unlike stronger acids, HClO is safe for handling and does not irritate the skin. This is particularly important in healthcare settings.
3. Eco-Friendly: HClO breaks down into harmless substances, minimizing its environmental impact. It decomposes into salt and water, making it a sustainable choice.
4. Versatile Applications: From household cleaners to water treatment, HClO finds use in many industries.
5. Non-Toxic: It poses minimal risk to human health and is safe to use in food preparation areas.
How Does HClO Work?
Let’s explore the mechanics behind HClO's effectiveness as a disinfectant.
Mechanism of Action
· Penetration: HClO penetrates microbial cells, damaging their structures and effectively killing them.
· Oxidative Stress: HClO causes oxidative stress in bacteria and viruses, leading to cell death.
· Broad-Spectrum Efficacy: HClO is effective against a wide range of pathogens, including bacteria, viruses, and fungi. This versatility makes it invaluable for sanitization.
The Hypochlorite Ion Formula
Understanding the hypochlorite ion (ClO⁻) enhances our grasp of HClO.
· Conjugate Base: The hypochlorite ion (ClO⁻) is the conjugate base of HClO. When HClO donates a proton, it becomes ClO⁻.
· Role in Chemistry: This ion also exhibits disinfectant properties, particularly in water treatment. It is often used in the form of sodium hypochlorite (NaClO) in household bleach.
Hypochlorite Ion Stability
· Stability Factors: The stability of ClO⁻ can be influenced by pH and temperature. At lower pH levels, ClO⁻ is more stable, contributing to the effectiveness of HClO as a disinfectant.
Practical Applications of Hypochlorous Acid
HClO finds its way into many products and applications. Here are some common uses:
1. Household Cleaners: Many sanitizers and disinfectants contain HClO due to its powerful antibacterial properties.
2. Water Treatment: It’s a staple in keeping swimming pools clean and safe for swimming. HClO effectively kills harmful pathogens that thrive in pool water.
3. Healthcare: Used in wound care and surgical settings, HClO helps prevent infections due to its antibacterial properties.
4. Food Industry: HClO is often used to sanitize food contact surfaces and equipment, ensuring food safety.
5. Agriculture: Farmers use HClO to sanitize equipment and control pathogens in irrigation water.
6. Veterinary Care: It is employed in animal healthcare for disinfecting wounds and surgical instruments.
How to Generate Hypochlorous Acid
We often rely on an HOCl Generator for efficient HClO production. Here’s how it works:
1. Mix Water and Salt: Start by combining water and salt. This mixture is essential for the electrolysis process.
2. Apply Electrolysis: The generator applies an electrical current to produce HClO. This process facilitates the reaction between chlorine ions and water.
3. Store the Solution: You can store the generated solution for future use. Ensure proper storage conditions to maintain its efficacy.
Benefits of Using an HOCl Generator
· On-Site Production: Generating HClO on-site ensures freshness and potency, reducing the need for transport and storage.
· Cost-Effective: Producing HClO with a generator can be more economical than purchasing pre-made solutions.
· Custom Concentrations: Users can adjust the concentration of HClO based on specific needs, making it versatile for various applications.
Comparing Hypochlorous Acid with Other Acids
How does HClO stack up against other acids?
Versus Strong Acids
· Safety: Unlike strong acids like hydrochloric acid (HCl), HClO poses less risk to human health and safety.
· Versatility: HClO is suitable for use in sensitive environments, such as food preparation and healthcare.
Versus Other Weak Acids
· Efficacy: HClO outperforms some weak acids in antimicrobial effectiveness. For example, acetic acid (vinegar) is weaker in disinfecting power compared to HClO.
The Importance of Proper Use
Using HClO correctly maximizes its benefits. Here are some tips:
1. Follow Guidelines: Always adhere to recommended concentrations for safety. Overuse or misuse can lead to ineffective disinfection.
2. Monitor pH Levels: Keeping pH in the optimal range ensures HClO remains effective. Regular testing can help maintain the desired pH level.
3. Store Properly: Store HClO in dark, cool places to maintain stability. Exposure to light and heat can degrade its potency.
Debunking Myths About Hypochlorous Acid
There are many misconceptions about HClO. Let’s address a few:
· Myth 1: HClO is dangerous.
· Fact: HClO is safer than many strong acids and has a low irritation potential.
· Myth 2: All disinfectants are the same.
· Fact: HClO is unique due to its efficacy and safety profile.
· Myth 3: HClO has a strong odor.
· Fact: Hypochlorous acid has a mild odor compared to chlorine gas, making it more pleasant to use.
Real-World Examples of HClO Use
In Healthcare
Hospitals and clinics utilize HClO for disinfecting surfaces and instruments. It helps prevent healthcare-associated infections, protecting patients and staff alike.
In Food Processing
Food manufacturers use HClO to sanitize equipment and surfaces. This practice helps ensure food safety and extends shelf life by reducing microbial contamination.
In Water Treatment Facilities
Municipal water treatment plants often employ HClO for disinfection.
References
1. Centers for Disease Control and Prevention (CDC) - Disinfecting with Hypochlorous Acid. CDC Hypochlorous Acid Guide
2. Journal of Environmental Science and Health - The Role of HClO in Water Treatment. Journal of Environmental Science
3. Chemistry LibreTexts - Acid Strength and Dissociation. LibreTexts Chemistry
4. Environmental Protection Agency (EPA) - A Guide to Chlorination and Hypochlorous Acid. EPA Guidelines
5. World Health Organization (WHO) - Water, Sanitation, and Hygiene: Guidelines for Disinfection. WHO Disinfection Guidelines
6. International Journal of Environmental Research and Public Health - Safety and Efficacy of Hypochlorous Acid. IJERPH
7. American Journal of Infection Control - The Antimicrobial Properties of Hypochlorous Acid. AJIC
8. Journal of Food Protection - Use of Hypochlorous Acid in Food Safety. Journal of Food Protection
9. Journal of Hospital Infection - Hypochlorous Acid as a Disinfectant in Healthcare. Journal of Hospital Infection
10. Food and Chemical Toxicology - Toxicology of Hypochlorous Acid and Its Applications. Food and Chemical Toxicology
